Waters Lcms Qc Reference Standard

Clrw Biomedical Water Systems Milliporesigma

Analytical Reference Standards Perkinelmer

Order New Reversed Phase Qc Reference Material Waters

Using Mass Spectrometry To Identify And Quantify Contaminants In Water Samples Sciex Community

Validation And Uncertainties Evaluation Of An Isotope Dilution Spe Lc Ms Ms For The Quantification Of Drug Residues In Surface Waters Sciencedirect

Waters Acquity Qda Detector Used Lab Equipment Conquer Scientific
All specially formulated based on the expertise of Waters scientist.

Waters lcms qc reference standard. But as a practical matter, concentrated reference standard solutions of Sucrose last 3-6 months, and analogous solutions of 1, 4 Benzoquinone (pBQ) last about 2 months, assuming they are stored at appropriate temperatures in appropriate containers and protected from light (for pBQ. In fact, most manufacturers of benchtop LC/MS systems claim that users can be up and running within a week. Analytical/Chromatography > Standards & Reference Materials System Maintenance Alert:.
For trueness and precision (recovery and relative standard deviation, RSD) evaluation, blank matrix samples were spiked at 0.01, 0.02, or 0.05 mg/kg in five replicates during the method validation. The only difference is that the Vion Test Mix box contains two ampoules each of 1 mL, while the LCMS QC Reference standard box contains one autosampler vial with 0.5 mL. It is intended to be used as a system benchmarking standard.
This solution—including the analyte(s) and internal standard(s)—should be evaluated after instrument maintenance, a power outage, break in instrument vacuum, instrument tuning/calibration, and prior to sample analysis ( Table 1 ). Calibration curve standards were made at 0.10, 0., 1.00, 50.01, 100.03, 125.03 and 149. ng/mL respectively while quality control samples were prepared at four levels, viz. This standard provides a comprehensive reference for the use with LC-MS or MS instrumentation with a wide variety of conditions and methods.
MilliporeSigma has developed a range of solutions adapted to the needs of scientists performing LC-MS. Beilstein/REAXYS Number 1014. AJO-287 (Phenomenex) Eluent A:.
It is intended to be used as a system benchmarking standard. Remedial Action If Calibration or Quality-Control Systems Fail to Meet Acceptable. The products in the portfolio are all specially formulated based on the expertise of Waters scientist.
Quality control (QC) samples included three quantitative positive controls and three qualitative controls. Additional The LCMS QCRM is a 9 component mix of Acetaminophen, Caffeine, Sulfaguanidine, Sulfadimethoxine, Val,-Tyr-Val, Verapamil, Terfenadine, Leucine-Enkephalin and Reserpine packaged in a Waters vial. Below you will find links to the main solutions proposed for LC-MS applications:.
See the two attached certificates of analysis for the exact composition. Molecular Weight 608.68. Individual Components in the LCMS QC Reference Material Mix.
Quality Control (QC Materials) e. Milli-Q® IQ 7003/7005/7010/7015 System;. Standard and Sample Preparation.
The BioAccord System is a self-calibrating, self-optimizing, self-sufficient tool that promises its users simplified high performance liquid chromatography-mass spectrometry (LC-MS) analysis and high quality data. Reference standards of carbamazepine (CBZ), carbamazepine-10,11-epoxide (CBZ-E) and 10-hydroxy-10,11-dihydrocarbamazepine (10-OH-CBZ), were purchased from Cerilliant (Round Rock, Texas), deuterated internal standards (ISTD), CBZ – D 8, CBZ-E – D 8 and 10-OH-CBZ – D 4, were from Toronto Research Chemicals (North York, Canada) ().For the production of calibrator levels a multi individual. Reserpine Standard for LC-MS analytical standard, for LC-MS Synonym:.
The Quality Control (QC) Reference Material portfolio is a unique collection of standards and mixtures. While both the QC samples and study samples might be expected to be spiked with internal standard (for a typical LC/MS/MS method) it must be recognized that the QC samples are also spiked with the analyte(s) of interest during their initial preparation. LCMS QC Reference Standard The LCMS QC Reference Standard is designed for use with high performance MS's like Synapt&.
50, v/v) to produce standard solutions with concentrations of 4, 10, 25, 50, 100, 150, 250, and 400 ng/mL. This standard provides a comprehensive reference for the use with LC/MS or MS instrumentation with a wide variety of conditions and methods. MS Qual/Quant QC Mix Proteomics MRM LC-MS Calibration Standard;.
XTerra MS C18 3,5 µm, 3,0 x 100 mm (Waters) Security Guard:. The LCMS QC Reference Material is a 9 component mix of Acetaminophen, Caffeine, Sulfaguanidine, Sulfadimethoxine, Val,-Tyr-Val, Verapamil, Terfenadine, Leucine-Enkephalin and Reserpine packaged in a Waters vial. Calibration standards and quality controls Calibration standards were prepared from 0.2 ml of drug-free serum by spiking with diluted methanolic working solutions (10 μl) of a mixture of THC, THCOH, and THCCOOH to obtain final concentrations of 1.0, 3.0, 6.0, 12.5.
WAT) today reinforced its commitment to advancing biotherapeutic characterization technology at the Well. The LCMS QC Reference Standard is designed for use with high performance MS's like Synapt G2-S, Xevo G-S QTof. Stocks and working standards were stored at 4°C under mostly dark conditions when not in use.
/PRNewswire/ -- Waters Corporation (NYSE:. Four QC levels were prepared at 50, 150, 750, and 4,000 ng/mL. For example, at 50% relative abundance, the.
Most of the suppliers of solutions specify expiry dates. LC-MS/MS Method for the determination of NDEA and NMDA in Valsartan, Irbesartan and Losartan APIs and finished dosage forms (developed on a AB Sciex QTrap 5500):. LCMS QC Reference Standard The LCMS Quality Control (QC) Reference Material is designed for use with high performance MS's like Synapt G2-S and Xevo G-S QTof.
Confidence in your results. 4 shows NPyr LC–MS/MS chromatograms corresponding to a reference standard of 5 μg L −1 (4a) and low level QC of the tap water sample 3 (10 ng L −1) (4b). After system suitability runs, standards were selected from E&L studies performed on a Q-TOF system for routine analysis of DP1.
When used consistently and control charted, the QC Reference Material will provide:. The LC/MS separation method was developed using these standards in positive and negative SCAN modes. CMS’s official document that provides guidelines for interpreting the CLIA regulations, as well as suggest probes for inspectors to use when reviewing a laboratory.
LC/MS/MS conditions LC/MS/MS analyses were conducted using 1290 Infinity II LC systems (1,0 bar) coupled to 6490 triple quadrupole LC/MS. Due to planned maintenance of our internal systems, web functionality including order placement, price and availability checks and SDS display will not be available for Asia and several European countries from Saturday, November 7th at 2:30 CET until Sunday, November 8th at 7:00 AM CET. Quantitative LC-MS Guide, 1st Ed.
The linearity range was 50–5000 ng/mL. Specific, sensitive analysis of PEth 16:0/18:1 in whole blood was obtained due to the unique selectivity and retention of the Raptor FluoroPhenyl column. The method established in this validation study provides accurate analysis of phosphatidylethanol (PEth) using a simple protein precipitation procedure and a fast 3.5 minute LC-MS/MS gradient run.
It is intended to be used as a system benchmarking standard. China / 简体中文 Japan / 日本語 India / English United Kingdom / English Germany / Deutsch France / Français. Individual Components in the Quad LCMS QC Reference Material Mix.
The mass spectra of the standards obtained from the SCAN data were added as reference spectra in the processing method. Calibration standards and quality control (QC) samples were prepared by spiking (2 % total volume of blank plasma) blank plasma with stock solution. Empirical Formula (Hill Notation) C 33 H 40 N 2 O 9.
Uncertainty of the results of a measurement expressed as a standard deviation. Major Instrumentation and Other Equipment. Standard and control solutions were vortexed for one minute, and 0 µl aliquots were transferred into 7 ml glass culture tubes and stored at -°C until used.
Key Applications QA/QC engineers can use LC/MS for most in-process chemical analyses, including:. Both, peak areas and Q/q ratios were similar for the reference standard and for the spiked tap water. The LCMS QC Reference Material is a 9 component mix of Acetaminophen, Caffeine, Sulfaguanidine, Sulfadimethoxine, Val,-Tyr-Val, Verapamil, Terfenadine, Leucine-Enkephalin and Reserpine packaged in a Waters vial.
However, in many applications it is necessary to isolate the target analyte from. (3β, 16β, 17α, 18β, α)-11,17-Dimethoxy-18-(3,4,5-trimethoxybenzoyl)oxyyohimban-16-carboxylic acid methyl ester, Reserpine CAS Number 50-55-5. There is no difference in composition between Vion Test Mix () and Waters LCMS QC Reference standard.
The LCMS QCRM is a 9 component mix used to provide a comprehensive reference standard for use with LC/MS or MS instrumentation with a wide variety of conditions and methods. ISO State Operations Manual, SOM. The Quad LCMS QC Reference Material is an 8 component mix used to provide a comprehensive reference standard for use with Waters Quad detectors, like Xevo®TQD and SQD, with a wide variety of conditions and methods.
50 µl of the IS working solution was added to 0 µl plasma sample, calibration standard, or quality control (QC) samples in a 7 mL culture tubes and vortexed. Intermediate standard solution of 100 ng/mL was prepared in water using commercially available nitrosamine reference standards. The Neutrals QC Reference Material is designed for use with any chromatographic systems containing a UV detector.
The Quad LCMS QC Reference Material is designed for use with any chromatographic systems containing a Quad MS such as the SQD, TQD, Xevo TQ. Process and QA/QC engineers can now use these versatile instruments as easily as use liquid or gas chromatography. Human urine (alcohol free) was fortified with EtG and EtS in order to prepare calibration standards and QC samples.
Each level was spiked with two internal standards NDMA-D6 & NDEA-D10 to obtain a resultant. Four QC levels were prepared at 50, 125, 700, and 4000 ng/mL. The concentration of each analyte is determined using the integrated peak area and internal standard technique.
C18 4 x 3,0 mm, Part No:. Milli-Q® IQ 7000 Water Purification System;. LC/MS Sample Preparation c.
As additional jurisdictions legalize Cannabis products and the variety and complexity of these products surpass the classical dried plant material, appropriate methods for. Quantitative controls were prepared by spiking blank matrix with MG to final concentrations of 3.00, 75.00 and 400.00 ng/mL. Linearity standards preparation Linearity standards of mixed nitrosamines were prepared from 0.25 ng/mL to 100 ng/mL levels.
The National Institute of Standards and Technology (NIST), The BioCollective (TBC), and the North America Branch of the International Life Sciences Institute (ILSI North America) are collaborating to extend NIST’s efforts to develop a Human Whole Stool Reference Material for the purpose of method harmonization and eventual quality control. The compounds in this mix give a mixture of responses in ESi (+-) and APCi+. LCMS QC Reference Standard The LCMS QC Reference Standard is designed for use with high performance MS's like Synapt&.
LC-MS Solvents and LC-MS Buffers & Reagents and LC-MS Accessories. Methanol/water (6:6:4, v/v/v)ofwhich5μl was injected into the LC-MS/MS system. Water LCMS grade Eluent B:.
Analyte-free pooled human urine (BioIVT) was fortified with d- and l-amphetamines and d- and l-methamphetamines (Cerilliant) to prepare seven calibration standards and four QC samples. These products allow the user to evaluate and benchmark their LC and LC-MS chromatography system before analysis of critical analytes. It is intended to be used as a system benchmarking standard.
A system suitability test (SST) uses a reference solution to verify performance of the LC-MS/MS analytical system. The concen-tration of calibration standards ranged from 50–5,000 ng/mL for both analytes. This standard provides a comprehensive reference for the use with LC/MS or MS instrumentation with a wide variety of conditions and methods.
By comparing retention times and signals produced by unique mass transitions to retention times and reference signals for matrix-matched calibration standards acquired under identical LC-MS/MS conditions. As an example, Fig. Uic and simple LC-MS/MS method for the determination of simvastatin in human plasma 545 Preparation of standard and quality control (QC) samples A stock solution (1 mg/mL) of simvastatin was diluted with acetonitrile:.
This standard mix should be used to confirm the benchmark performance of your Preparative/purification system. Calibration Standards and Quality Control Samples. COTININE IN SERUM ERAT-DLS Table Of Contents v 11.
Due to planned maintenance of our internal systems, web functionality including order placement, price and availability checks and SDS display will not be available for Asia and several European countries from Saturday, November 7th at 2:30 CET until Sunday. Stability and solubility constraints often. The mix contains only neutral compounds.
Find Sigma-Aldrich-MSQC1 MSDS, related peer-reviewed papers, technical documents, similar products & more at Sigma-Aldrich. The ion ratio must be within ± 10% of the average of the reference standards in a given analytical run as calculated by addition and subtraction. An LC/MS/MS is a liquid chromatograph (LC) system coupled with a quadrupole mass spectrometer (MS).
Acceptability of the batch. Earlier in 19, Waters Corporation announced their BioAccord LC-MS System for biopharmaceutical analysis. In the past 50 years, Cannabis sativa (C.
When analyzing with LC/MS/MS, the LC separates compounds by conventional chromatography on a column.

Highest Purity Solvents For Uhplc Ms Analysis Sigma Aldrich
Pdf Optimizing Lc Ms Ms Determination Of Microcystin Toxins In Natural Water And Drinking Water Supplies

Waters Oligonucleotide Analysis Solutions
2
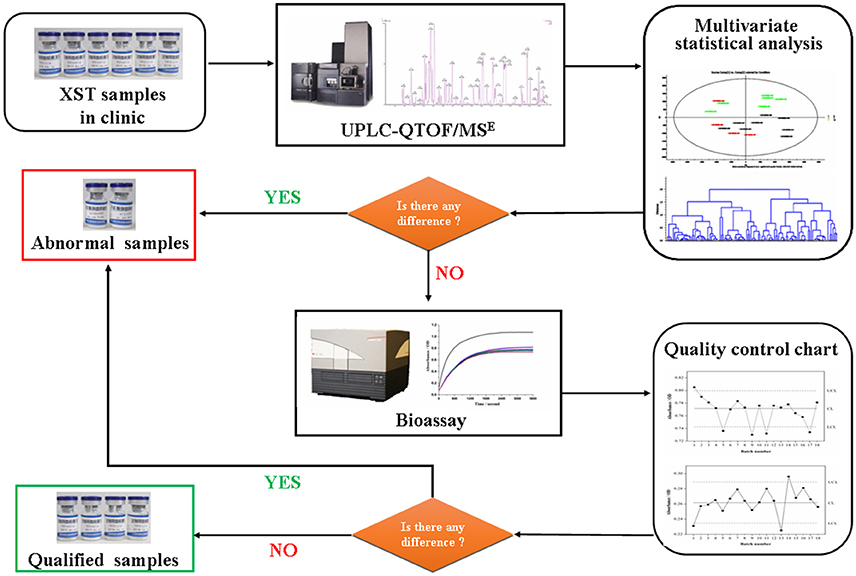
Frontiers Uplc Qtof Mse And Bioassay Are Available Approaches For Identifying Quality Fluctuation Of Xueshuantong Lyophilized Powder In Clinic Pharmacology
Qcloud A Cloud Based Quality Control System For Mass Spectrometry Based Proteomics Laboratories

Z Atwxi8uavpcm
Www Waters Com Webassets Cms Support Docs en Pdf

Ms Leucine Enkephalin Kit Analytics Shop Com
Analytical Standards And Reagents Waters
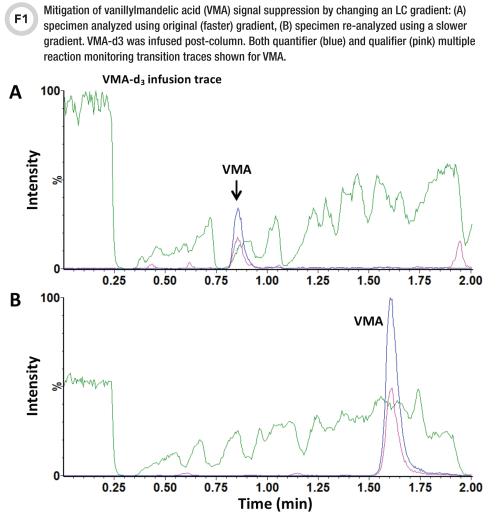
Interference Testing And Mitigation In Lc Ms Ms Assays cc Org

Chromatography Data Systems Perspectives Principles And Trends Chromatography Online
Http Www Waters Com Webassets Cms Library Docs en Pdf
2
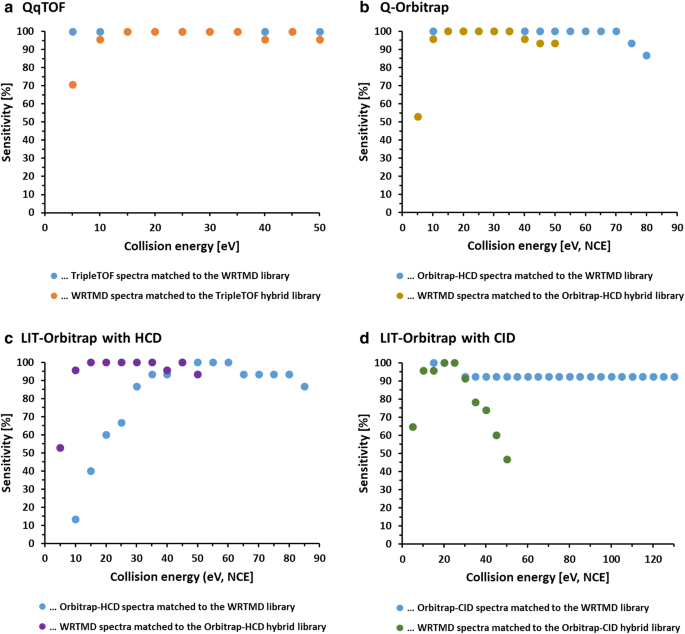
A European Proposal For Quality Control And Quality Assurance Of Tandem Mass Spectral Libraries Environmental Sciences Europe Full Text

Quality Control Reference Materials Waters

Bioanalysis Bioanalytics Bioanalytical Method Validation And Development Northeast Biolab
Http Www Crl Pesticides Eu Library Docs Cf Fp417 1 3 udgave engelsk version Pdf

Development Of An Uplc Ms Ms Method For Quantification Of Intact Igf I From Human Serum Bioanalysis

Quality Control Reference Materials Waters
Qcloud A Cloud Based Quality Control System For Mass Spectrometry Based Proteomics Laboratories
Http Www Waters Com Webassets Cms Library Docs en Pdf
Http Www Waters Com Webassets Cms Library Docs en Pdf

Analytical Standards And Reagents Waters

Tqncyz0afpov M

The Highs And Lows Of Gc Ms In Essential Oil Analysis Tisserand Institute

A Quality Control Of Proteomic Experiments Based On Multiple Isotopologous Internal Standards Sciencedirect
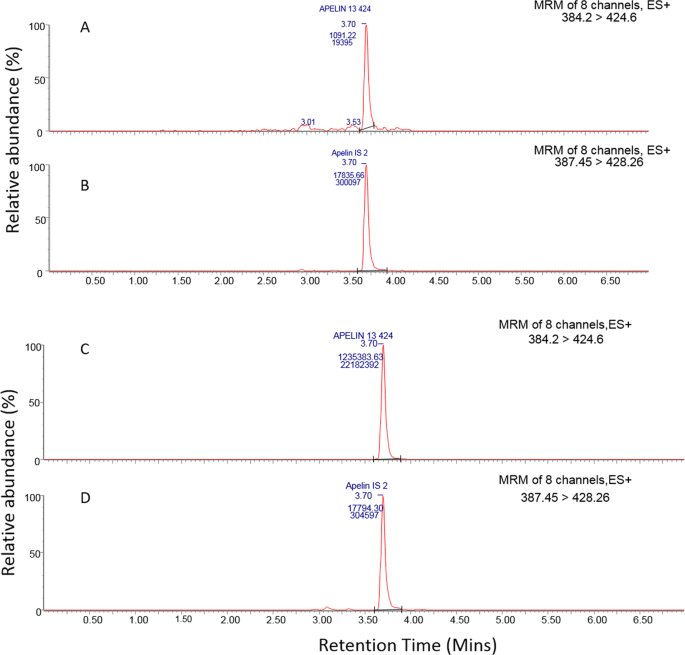
Development And Validation Of An Lc Ms Ms Method For Detection And Quantification Of In Vivo Derived Metabolites Of Pyr 1 Apelin 13 In Humans Scientific Reports

Alliance Hplc System Waters Corporation Evisa S Instruments Database
Supelco Certified Reference Materials Analytical Standards Choose The Right Reference Materials To Fit Your Needs From A Comprehensive Portfolio

Pierce Bsa Protein Digest Ms Grade

Pharmaceutical Secondary Standards Sigma Aldrich
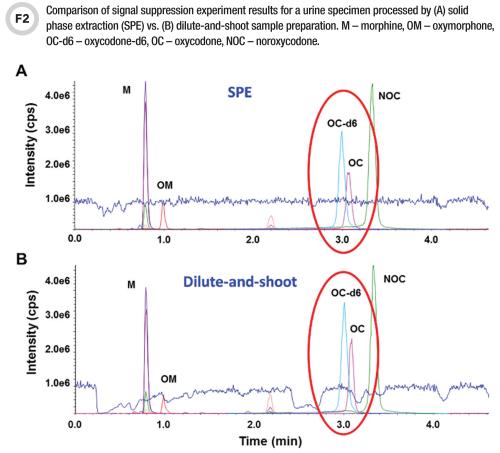
Interference Testing And Mitigation In Lc Ms Ms Assays cc Org

Mass Spectrometric Strategies For The Investigation Of Biomarkers Of Illicit Drug Use In Wastewater Hernandez 18 Mass Spectrometry Reviews Wiley Online Library

Analytical Standards Certified Reference Materials Sigma Aldrich
Analytical Standards And Reagents Waters
2

Analytical Standards And Reagents Waters

Water For Lc Ms Application Water Purification Milliporesigma

Cannabis Testing Is An Exact Science Regulations Are Not 19 06 24 Food Engineering

Waters Acquity Qda Detector Used Lab Equipment Conquer Scientific
Http Www Waters Com Webassets Cms Library Docs en Pdf
Http Www Waters Com Webassets Cms Library Docs en Pdf
Http Www Waters Com Webassets Cms Library Docs en Pdf

Aquastar Certified Reference Materials For Karl Fischer Titration Sigma Aldrich

Supelco Certified Reference Materials Analytical Standards Choose The Right Reference Materials To Fit Your Needs From A Comprehensive Portfolio
2
Http Www Waters Com Webassets Cms Library Docs en Pdf
Http Www Waters Com Webassets Cms Support Docs en Pdf

Measurement Of Vitamin D Metabolites By Mass Spectrometry An Analytical Challenge Zelzer Journal Of Laboratory And Precision Medicine

Ms Dial Tutorial Mtbinfo Github Io
Http Www Waters Com Webassets Cms Support Docs en Pdf

Quality Control Reference Materials Waters
Www Agilent Com Cs Library Applications 5991 64en Enviro non Target screening comp Pdf
Http Www Waters Com Webassets Cms Library Docs en Pdf

Quad Lcms Qc Reference Material Waters
Http Www Waters Com Webassets Cms Library Docs en Pdf

Waters Asr Catalog Waters Quality Control Reference Materials
Http Www Waters Com Webassets Cms Library Docs en Pdf

Order New Lcms Qc Reference Standards Waters
Http Www Waters Com Webassets Cms Library Docs en Pdf
Http Www Waters Com Webassets Cms Library Docs en Pdf
Http Www Waters Com Webassets Cms Library Docs en Pdf
Www Waters Com Webassets Cms Support Docs en Pdf
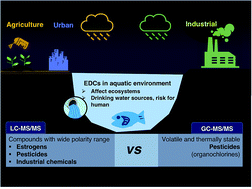
Comparison Of Gc Ms Ms And Lc Ms Ms For The Analysis Of Hormones And Pesticides In Surface Waters Advantages And Pitfalls Analytical Methods Rsc Publishing

Bioaccord Lc Ms Making Mass Spectrometry Available To The Masses Technology Networks

Assessing The Chromatographic Performance Of Small Polar Compounds When Using Hilic Based Lc Ms Chromatography For Metabolomic Studies Waters

Waters Asr Catalog Waters Quality Control Reference Materials
Eco Friendly Lc Ms Ms Method For Analysis Of Multi Class Micropollutants In Tap Fountain And Well Water From Northern Portugal Springerlink
Www Cerilliant Com Products 15 16 Cerilliant Analytical Reference Standards Catalog Pdf

Waters Asr Catalog Waters Quality Control Reference Materials

Waters Asr Catalog Waters Lcms Qc Reference Materials

Quantifying Auxin Metabolites In Young Root Tissue Of
Liquid Chromatography Mass Spectrometry Wikipedia
Http Www Waters Com Webassets Cms Support Docs en Pdf

Waters Oligonucleotide Analysis Solutions

Waters Tqd Q Premier Xe Q Micro Kit Analytics Shop Com
Http Www Waters Com Webassets Cms Library Docs en Pdf
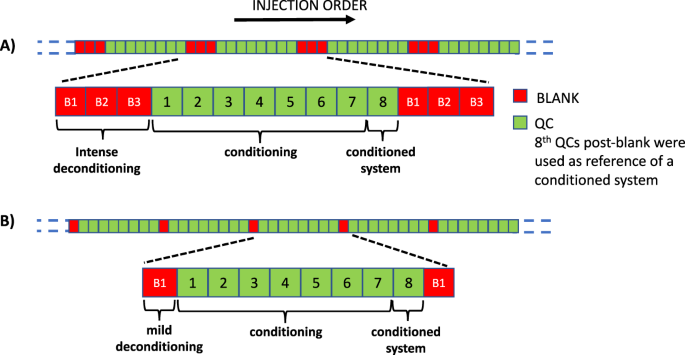
Monitoring Of System Conditioning After Blank Injections In Untargeted Uplc Ms Metabolomic Analysis Scientific Reports
Www Agilent Com Cs Library Applications 5991 64en Enviro non Target screening comp Pdf
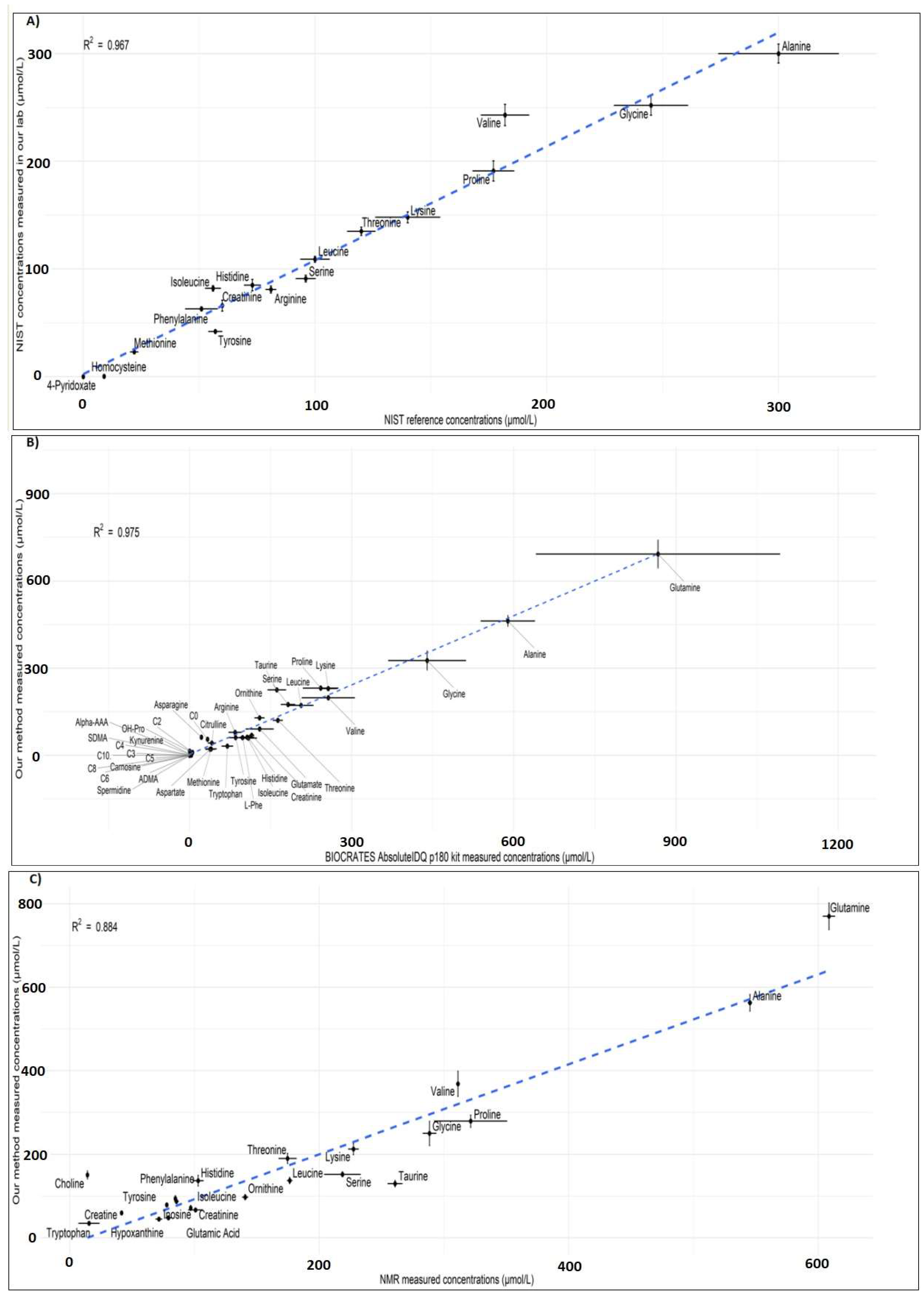
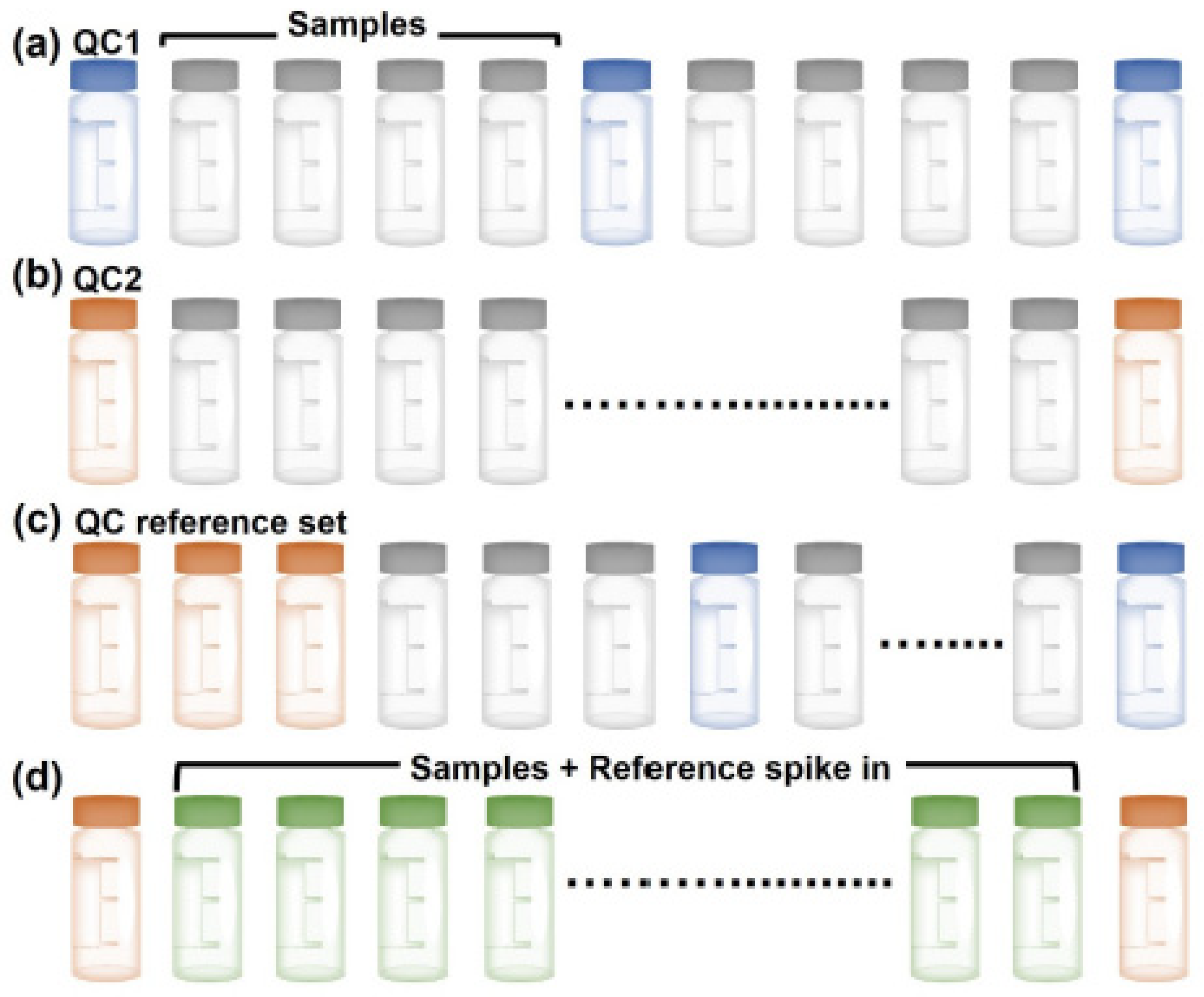
Metabolites Free Full Text Validation And Automation Of A High Throughput Multitargeted Method For Semiquantification Of Endogenous Metabolites From Different Biological Matrices Using Tandem Mass Spectrometry Html

Analytical Reference Standards Perkinelmer

Search Standards Reference Standards Chromatography Products At Restek Com

Chromatography Data Systems Perspectives Principles And Trends Chromatography Online

Pesticide Residue Testing Accurate Standard Preparation

Quantification Of Plasma Remdesivir And Its Metabolite Gs Using Liquid Chromatography Coupled To Tandem Mass Spectrometry Application To A Covid 19 Treated Patient In Clinical Chemistry And Laboratory Medicine Cclm Volume 58 Issue 9
Molecules Free Full Text Mass Spectrometry Advances And Perspectives For The Characterization Of Emerging Adoptive Cell Therapies Html
Http Www Waters Com Webassets Cms Library Docs en Pdf

Multi Attribute Monitoring Of Antibody Modifications By Semi Automated Liquid Chromatography Mass Spectrometry Peptide Mapping American Pharmaceutical Review The Review Of American Pharmaceutical Business Technology

Phenomenex Hplc Part Luna 5 µm C18 2 100 A Lc Column 250 X 4 6 Mm Ea Part 00g 4252 E0
Care And Use Manual Waters Lcms Quality Control Reference Materials User Manual Page 3 3
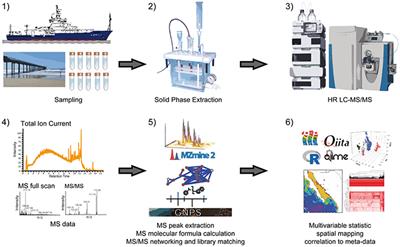
Frontiers High Resolution Liquid Chromatography Tandem Mass Spectrometry Enables Large Scale Molecular Characterization Of Dissolved Organic Matter Marine Science
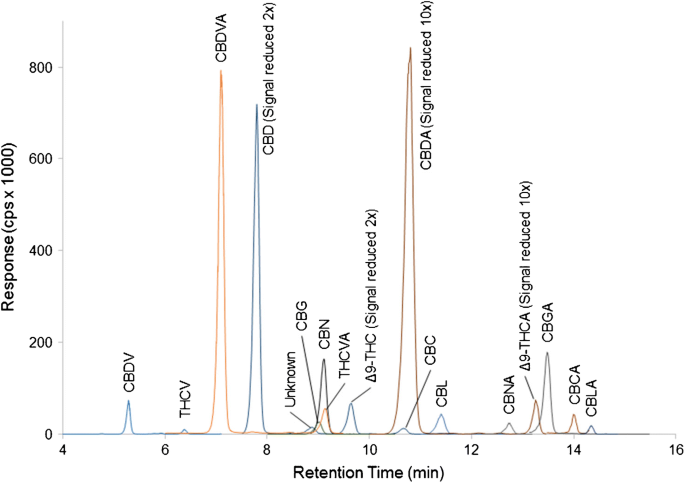
Quantitative Determination And Validation Of 17 Cannabinoids In Cannabis And Hemp Using Liquid Chromatography Tandem Mass Spectrometry Springerlink
Www Waters Com Webassets Cms Support Docs en Pdf



